
Regulation of intracellular pH, gas channels
We have three major research areas (see below). These evolved from our longstanding interest in intracellular pH (pHi) homeostasis, which is critically important because virtually every biological process—cell division, metabolism, action of channels/transporters/structural proteins—depends on pHi. Our three projects interact philosophically and technically. Philosophically, pHi homeostasis depends on HCO3− and H+ transport across cell membranes, which in turn depends on pH, [CO2] and [HCO3−] in the extracellular (o) fluid. The kidneys regulate pHo by regulating [HCO3−]o—and they do this by using novel sensors to sniff CO2 and HCO3− in the blood. The lungs, controlled by brainstem neurons, regulate pHo by regulating [CO2]o. The whole system depends on movements of CO2 (and other gases) through gas channels in the cell membrane. Technically, our projects exploit technologies ranging from structural biology, through molecular and cell physiology, to whole tissues and organisms.
Bicarbonate handling by the proximal tubule
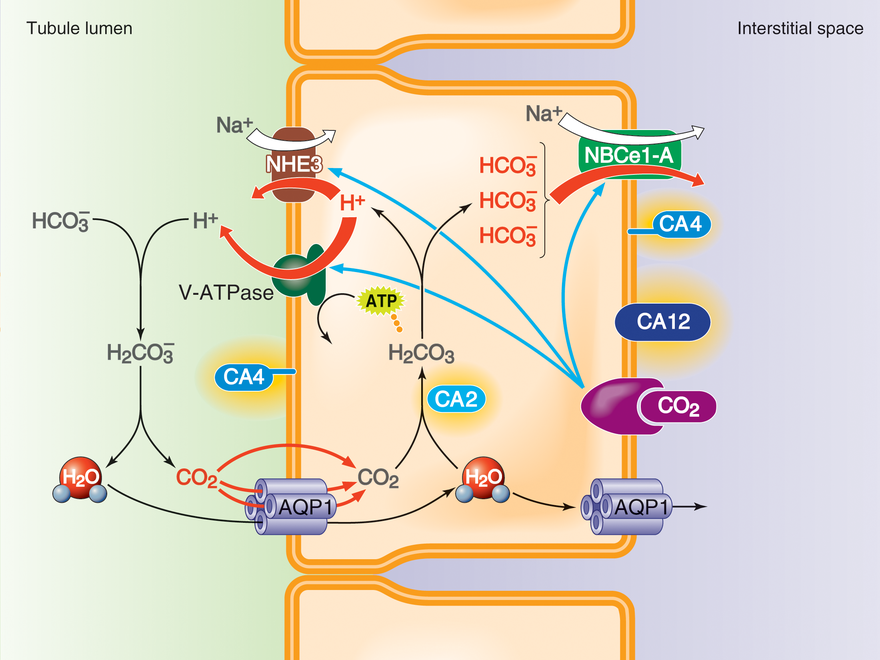
The renal proximal tubule is a microcosm of the problems that we study in the Lab. Each day, the glomeruli of a human typically filter—from the blood to the tubule fluid—nearly a half kg of baking soda (NaHCO3). The kidney must secrete into the lumen enough H+ to titrate (and thereby reclaim) virtually all of the filtered bicarbonate as well as to rid the body of the fixed acid produced by metabolism.
Homeostasis of intracellular pH
As a graduate student, I imposed acute intracellular acid loads and found that the sudden pHi decrease is followed by a recovery—the first demonstration of pHi regulation. Later work showed that this recovery is mediated by a Na+-driven Cl-HCO3 exchanger (NDCBE)—the first documented pHi regulator. As a fellow, we identified the electrogenic Na/HCO3 cotransporter (NBCe1), which not only regulates pHi but also mediates HCO3− movement of across many epithelia.
Since cloning the cDNA encoding NBCe1, our group has studied the molecular mechanism of Na+-coupled HCO3− transporters (NCBTs)—members of the SLC4 family of transport proteins. For example, emerging data indicate that NBCe1 and NDCBE—expressed in Xenopus oocytes—transport CO3= rather than HCO3−. Carbonic anhydrases do not change transport rate but stabilize surface pH. Chimera making shows that extracellular loop #4 is critical for determining electroneutrality vs electrogenicity. Certain splice variants have autoinhibitory or autostimulatory domains. The large cytoplasmic N terminus of NBCe1 binds to and thereby activates the transmembrane domain. Finally, we are producing large quantities of NBC-related proteins to study their molecular biophysical properties.
Sensors for extracellular CO2 and HCO3−
While studying isolated, perfused proximal tubules (PTs), we noticed that adding CO2/HCO3− to the PT's basolateral (BL or blood) side causes pHi to increase, reflecting stimulated H+ extrusion across the apical membrane (facing lumen). To elucidate the mechanism, we invented out-of-equilibrium (OOE) CO2/HCO3− solutions, which allow us to vary [CO2]BL, [HCO3−]BL, and pHBL—one at a time. We found that isolated increaes in [CO2]BL or isolated decreases in [HCO3−]BL stimulate H+ secretion into the tubule lumen (JH) . Surprisingly, acute changes in pHBL have no effect on JH! Thus, the tubule regulates blood pH by sensing not pH but the two major blood buffers.
The signal-transduction system linking basolateral CO2 and HCO3− to apical H+ extrusion involves: (1) Apical AT1 (angiotensin II, ANG II) receptors and ANG II that the tubule secretes into the lumen. (2) Tyrosine phosphorylation. Inhibitors of ErbB receptor tyrosine kinases block responses to basolateral CO2 or HCO3−. The same is true for knockout of receptor protein tyrosine phosphatase γ (RPTPγ), in which the extracellular ligand-binding region is an inactive carbonic-anhydrase-like domain (CALD). We are testing the hypothesis that the CALD is the sensor for extracellular CO2 and HCO3−. We are also using biochemical approaches to explore signal transduction.
Gas channels
While studying pHi regulation in gastric parietal cells we found that the apical membrane has no detectable permeability to either CO2 or NH3—the first documented gas-impermeable membrane. This led us to rethink “Overton’s rule” and to describe the first gas channel aquaporin 1 (AQP1), which is not only permeable to H2O but also CO2. Others later showed that AQP1 is permeable to NH3, and that the rhesus (Rh) proteins are permeable to CO2 and NH3.
We developed an approach for using a large pH microelectrode to monitor the extracellular surface pH (pHS) on Xenopus oocytes exposed to CO2 or NH3. Initial pHS transients are indices of CO2 or NH3 permeability. Studying a wide range of AQPs and Rh proteins, we see that each has a characteristic CO2/NH3 permeability ratio—the first example of gas selectivity. Preliminary work suggests that NH3 moves only through AQP or Rh monomers, whereas CO2 generally prefers the central pore of the oligomer. Preliminary stopped-flow work with erythrocytes (RBCs) indicates that certain inhibitor combinations can block 75% of O2 efflux from RBCs—the first evidence for O2 channels.
| Major Research Areas |
|---|
| Gas Channels, Membrane Transport, pH Regulation |
| Disciplines |
| Cellular Modelling and Simulation, Electrophysiology, Physiological Phenotyping, Signal Transduction |
| Organ Systems |
| Cardiovascular System, Nervous System, Renal System, Respiratory System |
| Diseases |
| Acid-Base Disturbances, Hypertension, Hypoxia |
| Major Techniques |
| Cell Culture, Confocal Microscopy, Electrophysiology, Ex Vivo Functional Analysis, Gel Electrophoresis/Western Blots, Ion Transport, Ion-Sensitive Microelectrodes, Patch Clamping, Protein Crystallization, Protein Expression, Protein-Protein Interactions (Biocore), Quantitative Fluorescence Imaging, RNA Isolation and Gene Expression Analysis, RT-PCR, Two-Electrode Voltage-Clamp Technique |
| Genes |
| CAR12 (CA XII), CAR2 (CA II), CAR4 (CA IV), CAR9 (CA IX), PTPRG (RPTPg), PTPRZ (RPTPz), SLC4A1 (AE1), SLC4A10 (NBCn2 (NCBE)), SLC4A11 (BTR1), SLC4A2 (AE2), SLC4A3 (AE3), SLC4A4 (NBCe1), SLC4A5 (NBCe2), SLC4A7 (NBCn1), SLC4A8 (NDCBE), SLC4A9 (AE4) |