Proteostasis maintenance of membrane proteins in health and disease
My laboratory aims to understand protein homeostasis (proteostasis) of ion channels. They are major drug targets; loss of their proteostasis and thus function leads to numerous diseases, including neurological, neurodegenerative, and cardiovascular diseases. To function, ion channel proteins must fold into their native structures and assemble properly in the endoplasmic reticulum (ER) for subsequent trafficking to the plasma membrane in a fully functional state. Variations in a given protein could lead to protein misfolding and excessive degradation through the ER-associated degradation (ERAD) pathway or autophagy, and thus a significantly lowered concentration of proteins in cell membranes and loss of function.
Currently, in my laboratory, we focus on studying neurotransmitter receptors, including gamma-aminobutyric acid type A (GABAA) receptors, the primary inhibitory ion channels, and N-methyl-D-aspartate (NMDA) receptors, the major excitatory ion channels in the human brain. They mediate the inhibition-excitation balance in the mammalian central nervous system. Their dysfunction leads to epilepsies, autism, and other neurodevelopment diseases.
We explore how molecular chaperones, folding enzymes, degradation factors, and trafficking factors, coordinate to facilitate membrane protein folding, assembly, degradation, and trafficking.
We also use small molecules, incluing proteostasis regulators and pharmacological chaperones, to correct misfolded membrane proteins, as a therapeutic strategy to treat corresponding diseases.
Proteostasis maintenance of GABAA receptors
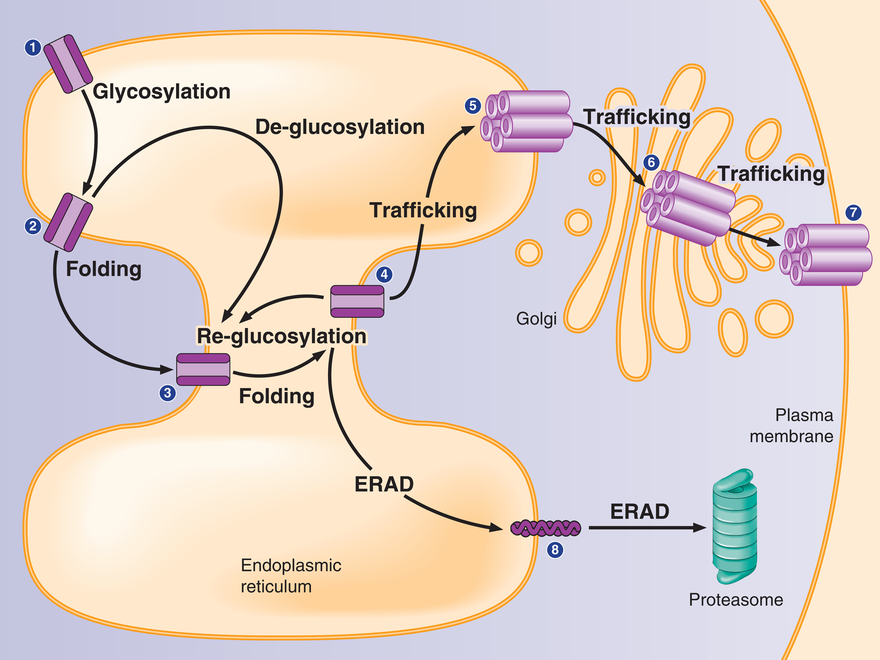
Ion channel proteins maintain a delicate balance between protein synthesis, folding, assembly, trafficking and degradation. Mutant membrane proteins are subjected to excessive endoplasmic-reticulum associated degradation. Molecular chaperones and ERAD factors play a key role in regulating their proteostasis.
Understanding the molecular mechanism underlying ion channel folding, assembly and degradation
Because the activity of membrane proteins is coupled to other proteins in complicated cellular interaction networks, the molecular mechanism of ion channel folding and degradation in the cell has been elusive. Using representative membrane proteins, we aim to reveal the protein homeostasis network that control their folding, assembly, degradation, and trafficking and thus function using proteomics, cell biology, electrophsiology, and animal model approaches.
Ameliorating ion channel misfolding diseases
We focus on diseases resulting from protein misfolding and thus reduced membrane protein surface expression and compromised function. This defective protein trafficking has been reported to result in many diseases, including neurodegenerative diseases and cardiovascular diseases.
We focus on developing multiple strategies to discover small molecules that enhance proper protein folding in cells to ameliorate diseases. We also explore the mechanism of action of these small molecules: how they alter the signaling pathways associated with the protein homeostasis network for disease intervention. Ultimately, we aim to develop novel therapeutic strategies for diseases associated with protein misfolding.
| Major Research Areas |
|---|
| Ion Channels, Membrane Trafficking, Protein Folding, Protein Folding and Misfolding |
| Disciplines |
| Animal Models, Biochemistry, Cellular Biology, Drug discovery , Electrophysiology, Fluorescence Spectroscopy, Neuroscience, Pharmacology and Therapeutics, Protein folding and misfolding |
| Organ Systems |
| Nervous System |
| Diseases |
| Channelopathies, Epilepsy, Neurodegenerative Diseases, Neurological Disorders |
| Major Techniques |
| Cellular and Molecular Biology, Electrophysiology, Immunohistochemistry, In-vivo Animal Models, Mass Spectrometry |
| Genes |
| GABRA1 (), GABRB2 (), GABRG2 (), GRIN1 (None), GRIN2A (None), GRIN2B (None) |