
Regulation of epithelial ion transporters in health and disease
The vectorial movement of electrolytes Na and Cl across epithelia is a highly regulated physiological process that is vital for maintaining electrolyte and volume homeostasis. In epithelia lining the renal tubules and airway segments, two plasma membrane ion transporters play critical roles. One, a basolateral Na-K-Cl cotransport (NKCC1) is rate limiting for overall epithelial Cl and fluid secretion. Second, an apical Cl channel (CFTR, cystic fibrosis transmembrane conductance regulator) mediates energy-efficient movement of Cl from the cell into the lumen. Genetic abnormalities in NKCC1 and CFTR lead to a defined pathophysiology. Defects in CFTR processing, trafficking, regulation or transport capacity prevents secretion of fluid and electrolytes in airway epithelia, a process thought contributory to the lung pathogenesis in cystic fibrosis patients. Loss of NKCC1 leads to vestibular dysfunction and deafness, decreased GABA response, abnormal spinal cord Cl levels resulting in pain, reduction in salivation, and hypotension. In human airways, abnormal NKCC1 is linked to asthma and COPD.
In airway epithelia, NKCC1 supplies Cl for secretion; hence, manipulation of NKCC1 activity by infectious agents, mediators or drugs compromises baseline Cl secretion and response to hyperosmotic stress. I am dissecting signal transduction cascades involved in activation of NKCC1. My studies provided the first evidence for activation of NKCC1 cotransport by alpha-adrenergic agonists, hyperosmotic stress and intracellular Cl. Subsequent investigation of membrane-associated events, including activation of phospholipases A2, C, and D, their regulation by G proteins, and generation of lipid mediators, led to more detailed study of protein kinase C (PKC) regulation of cotransport. Using antisense oligonucleotides targeted to specific PKC isotypes, we obtained compelling evidence for a critical role for PKC-delta isoform in regulation of NKCC1. Our current studies focus on protein-protein interactions of protein kinases and phosphatases with the amino terminus of NKCC1. We have more recently identified binding of a small molecular weight cytosolic protein to the carboxyl terminus of NKCC1. We are now investigating how the protein complexes regulate NKCC1 activation and membrane expression using techniques from protein biochemistry, mass spectrometry, cell physiology, bioinformatics and molecular biology. One goal of this project is to determine how to NKCC1 activity can be manipulated to normalize Cl secretion.
Regulation of CFTR membrane expression and function.
Several multi-domain adaptor proteins have been identified as key regulators of CFTR membrane localization and activity. These adaptors integrate crucial functions including a) tethering of CFTR to the actin cytoskeleton and suppression of CFTR endocytosis; b) CFTR clustering with other membrane proteins, including receptors and channels/transporters; c) integrating signaling and acting as scaffolds for other CFTR regulatory proteins, including kinases. We have identified two adaptors, NHERF1 and filamin A (FlnA), as necessary for maintenance of physiological CFTR levels at the membrane. Loss of each of these adaptors or disruption of their interactions with CFTR causes a drastic reduction in membrane CFTR levels. This may be linked to enhanced endocytosis of CFTR after it is decoupled from the actin cytoskeleton. Loss of surface CFTR, however, may also be due to an inability of NHERF1- or filamin-interacting proteins to properly regulate CFTR when these adaptors are absent. The long-term goal of this project is to determine how CFTR-interacting proteins regulate the membrane localization and channel properties of CFTR. In addition, we want to determine how these CFTR-regulatory processes can be modulated to ameliorate pathologies caused by dysregulation of epithelial ion transport and hydration.
PKC signalling has been implicated in the regulation of the Cl channel CFTR but with little detailed information on how PKC controls CFTR function. Rapid activation of CFTR is achieved through stimulation of beta-adrenergic receptors. We have demonstrated a pivotal role for a PKC-epsilon isoform during activation of CFTR. Our subsequent and current studies focus on protein-protein interactions that explain PKC regulation of CFTR. We have shown a unique role for protein-protein interactions involving PKC-epsilon binding to RACK1 and binding of RACK1 to NHERF at the PDZ1 domain. More recently, we discovered direct binding of RACK1 to filamin-A, an actin-binding protein. Thus, RACK1 may form two adaptor complexes to selectively regulate PKCepsilon phosphorylation of its substrate target. Our long term goal is to identify and characterize the target substrate for PKC-epsilon and learn how modification of the substrate regulates activation of CFTR.
RACK1-nexus adaptors in the regulation of CFTR.
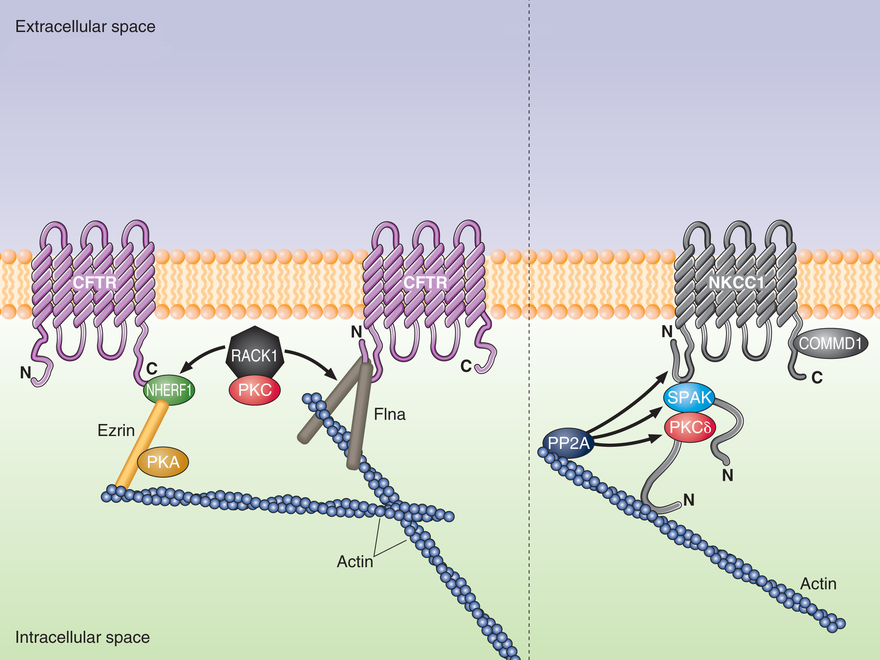
RACK1 interacts with filamin-A at the amino terminus of CFTR and with NHERF1 at the carboxyl terminus of CFTR. PKCepsilon is necessary for cAMP-activation of CFTR and is tethered to RACK1.