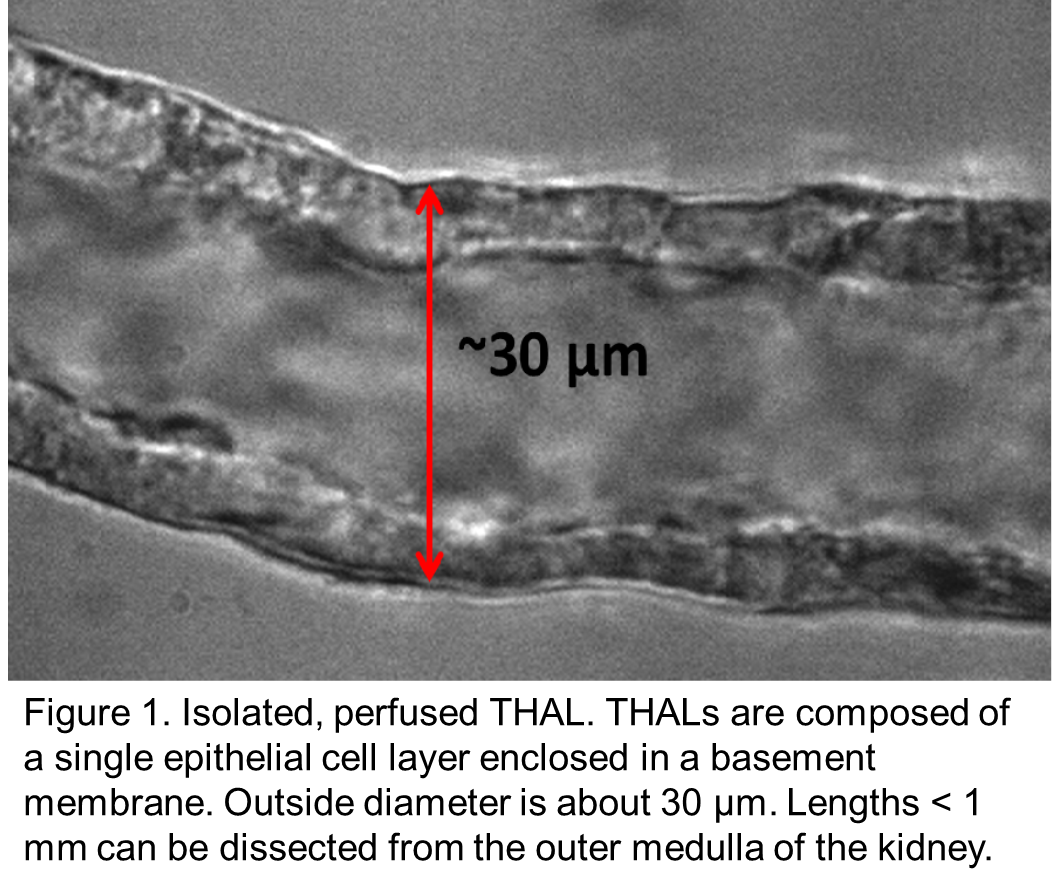
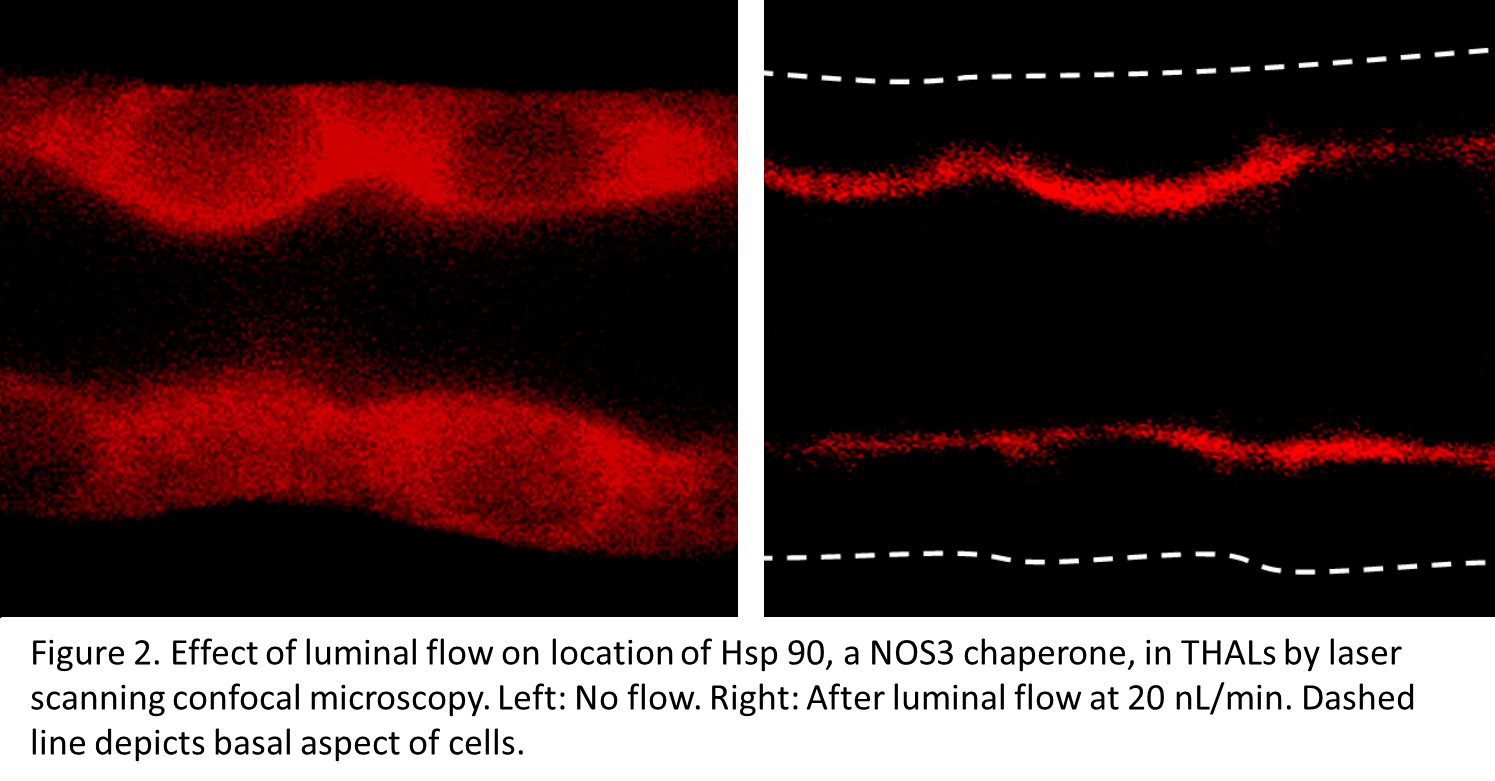
Inappropriate salt retention by thick ascending limbs causes salt-sensitive hypertension in humans and animals. Hypertension is the leading cause of “loss of health” world-wide. Superoxide (O2-) and nitric oxide (NO) are key regulators of thick ascending limb NaCl reabsorption and thus urinary Na excretion (UNaV), urinary volume (UV) and blood pressure.
Urine flow regulates nephron transport. Flow elevates wall stretch and shear stress which stimulate O2- production by NADPH oxidase 4 (NOX4) and NO production by NO synthase 3 (NOS3), respectively. We reported that flow-stimulated Na reabsorption depends on O2- and protein kinase C α (PKCα) but that flow-induced NO buffers flow-stimulated NaCl reabsorption by directly inhibiting NaCl transport and blunting flow-induced O2-. A high-salt diet increases thick ascending limb flow and we reported that it enhances thick ascending limb NO synthesis by increasing NOS3 expression and activity via phosphorylation of serine 1179 (S1179; a stimulatory site), and de-phosphorylation of threonine 495 (T495; an inhibitory site) modified by PKC. Thus, salt-induced increases in luminal flow prevent salt retention and increases in blood pressure under normal circumstances.
Mechano-transduction of flow-induced stretch and shear stress in epithelia may occur via TRPV4 channels and/or cilia with TRPV4-TRPP2 channel activation. We showed that flow-induced stretch stimulates TRPV4, O2- and increases in intracellular Ca (Cai). We also showed that TRPV4 mediates flow-induced increases in Cai and NO. Since cilia and TRPV4-TRPP2 channels sense shear stress, both may be involved in NO production. If so, stretch and shear stress may differentially activate TRPV4 and cilia/TRPV4-TRPP2 channels, respectively. This would allow cells to distinguish stretch- and shear stress-induced Cai increases. However, the roles of TRPV4, TRPP2 and cilia in flow-induced O2- and NO, and salt-sensitive hypertension are unclear.
In Dahl salt-sensitive rats (SS) an imbalance between O2- and NO favoring the former causes salt-induced increases in blood pressure; why this is the case is unknown. Our preliminary data show that stretch-induced TRPV4-dependent increases in Cai and flow-induced O2- production are greater in SS than salt-resistant (SR) thick ascending limbs; however flow-induced NO production is reduced. This decrease is NOT due to scavenging by O2- or differences in NOS3 expression. Our preliminary data also show that a high-salt diet augments the differences in flow-induced Cai and O2- between SS and SR thick ascending limbs, and causes a difference in NOS3 expression. Thus we hypothesize that SS thick ascending limbs display abnormally increased TRPV4 channel activity in response to salt-enhanced luminal flow causing abnormally elevated Cai and O2- production by NOX4. Chronically elevated O2- blunts the ability of flow to stimulate NO synthesis as a result of diminished salt-stimulated NOS3 expression, enhanced NOS3 phosphorylation at T495. These effects result in salt retention by thick ascending limbs and salt-sensitivity of blood pressure. Thus the ultimate cause of salt-sensitivity is a defect in mechano-transduction.
To test this hypothesis we will study the effect of raising flow on TRPV4 activity, Cai, O2-, PKC activity and NaCl reabsorption in SS and SR thick ascending limbs on normal and high-salt diets. We also will study the effects of altering TRPV4 expression on Cai and O2-, and blunting NOX4 and PKCα activity on O2- and Na transport at various flows. Next we will study the effect of flow on NO production and NO-induced inhibition of NaCl reabsorption in SS and SR thick ascending limbs from rats on normal and high-salt diets, and the effects of overexpressing dominant-negative PKCα and knocking down NOX4, TRPV4, TRPP2 and Ift88 (required for cilia). We will study NOS3 expression, NOS3 phosphorylation and BH4 levels. We will also measure cilia stiffness, Akt activation and NO production in single cells of isolated tubules. Finally we will study the effects of altering NOS3, NOX4, and TRPV4 and dominant-negative PKCα expression on blood pressure and renal function with and without bumetanide after an acute salt load in SS and SR on normal and high-salt diets.
Our unique hypothesis may provide a fundamental explanation for disparate data concerning the roles of NO and O2- in salt-sensitivity of blood pressure in SS.